VivaDiag Pro SARS-CoV-2 Ag Rapid Test
Antigen Test Results in just 15 minutes
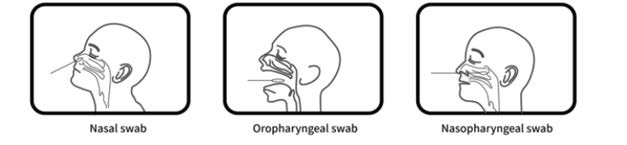
VivaDiagTM Pro SARS-CoV-2 Ag Rapid Test is for the rapid, qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in human nasal swab, oropharyngeal swab
or nasopharyngeal swab specimen specimen. The test is for in vitro diagnostic use only. For professional use only. It is intended for clinical laboratories and healthcare professional use only for point of care testing. Not for at home testing.
VivaDiagTM Pro SARS-CoV-2 Ag Rapid Test is based on immunochromatography technology. Each test device has one line of anti-SARS-CoV-2 antibody on the detection line (T line) and one line of anti-mouse IgG antibody on the quality control line (C line). When extracted specimen is added to the specimen well, it will react with the labeled antibody to form a complex, the mixture then migrates through the membrane by capillary action and interacts with the coated anti-SARS-CoV-2 antibody on the detection line. If the specimen contains SARS-CoV-2 antigen, the detection line will appear red indicating the SARS-CoV-2 antigen is positive. Otherwise, the test result will be negative. The test device also contains a quality control line C which should appear red for all valid tests. If the quality control line C does not appear, the test result will be invalid even if the detection line appears.
Each test kit contains test devices, sealed pouches (prefilled with 300 μL extraction solution), extraction tubes, extraction tube tips, tube stand, sterile swabs and package insert.
Clinical Validation
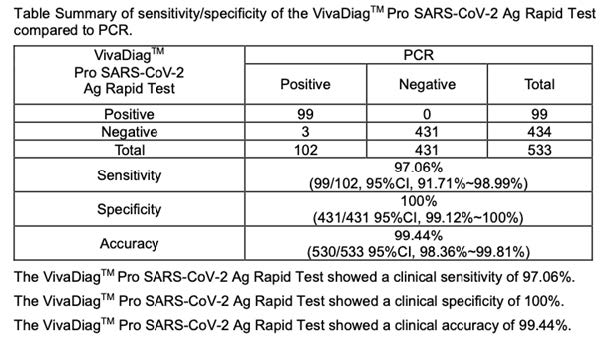
A total of 533 specimens were tested using the VivaDiagTM Pro SARS-CoV-2 Ag Rapid Test. These specimens were obtained by nasal swabs from symptomatic patients. The performance of the VivaDiagTM Pro SARS-CoV-2 Ag Rapid Test was compared to a commerialized molecular PCR assay. Table Summary of sensitivity/specificity of the VivaDiagTM Pro SARS-CoV-2 Ag Rapid Test compared to PCR.
 Quick Overview of Method:
Quick Overview of Method:
Specimen Collection
Nasal swab specimen (recommended)
It is important to obtain as much secretion as possible. Insert the sterile swab into one nostril. The swab tip should be inserted up to 2.5 cm (1 inch) from the edge of the nostril. Roll the swab 5 times along the mucosa inside the nostril to ensure that both mucus and cells are collected. Repeat this process for the other nostril to ensure that an adequate specimen is collected from both nasal cavities (use the same swab).
Specimen Handling
Freshly collected specimens should be tested as soon as possible. It is essential that correct specimen collection and preparation methods are followed.
1. Hold the sealed pouch vertically and let all extraction solution flow into the bulb. Break the tip and squeeze the bulb to dispense all extraction solution into the extraction tube.
2. Collect specimen refer to Specimen Collection.
3. Insert the swab with collected specimen into the extraction tube filled with extraction solution. Roll the swab 5 times while pressing the head against the bottom and side of the extraction tube. Remove the swab while squeezing the sides of the tube to extract the liquid from the swab. Try to release as much liquid as possible. Dispose the used swab as a biohazard waste.
4. Put on the tube tip.
5. Take out a test device from sealed foil pouch and put it on a clean and level surface.
6. Apply 3 drops (about 60 μL) of the extracted specimen into the specimen well. Please avoid bubbles during applying.
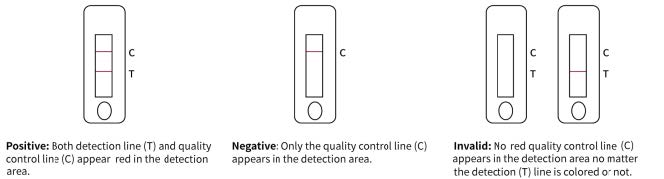
Reading Results
1. Positive Result. Both the quality control line C and the detection line T appear.
2. Negative Result. Only the quality control line C appears, with no other line appearing on the detection line.
3. Invalid Result. Quality control line C fails to appear indicating the test is invalid, no matter if the detection line appears or not. Collect a new specimen and perform another test with a new test device. est result at 15 minutes. Don’t read the result after 20 minutes.
Click HERE to download Clinical Study Report
Contact us to learn more about the VivaDiag Pro SARS-CoV-2 Antigen Rapid Test

